 December 4, 2020 11:13 am
Published by Fluidda
December 4, 2020 11:13 am
Published by Fluidda
Galapagos Announces Positive Topline Results in Phase 2 Study Using FRI Imaging Parameters While Investigating New Medication for IPF Patients. The PINTA trial was a randomized, double-blind, placebo-controlled trial investigating... View Article
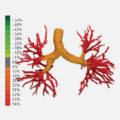 February 25, 2020 10:48 am
Published by Fluidda
February 25, 2020 10:48 am
Published by Fluidda
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with unclear etiology. IPF patients decline with heterogeneous trajectories. The ability to determine an individual’s disease course is limited to pulmonary function measurement... View Article
January 1, 2019 12:03 pm
Published by Fluidda webmaster
WU et al.
AJRCCM Issues > Vol. 199, No. 1 | Jan 01, 2019
May 28, 2018 12:45 pm
Published by Fluidda webmaster
Maher et al.
www.thelancet.com/respiratory Published online May 20, 2018


