Theoretical Model of the Pathophysiology of COVID-19 using FRI-data in EMCRIT podcast
During an EMCRIT podcast last week. Dr. Farid Jalali (Long beach CA) laid out a theoretical framework for the pathophysiology of the lung effects of COVID-19.
Take Home Points from this podcast were:
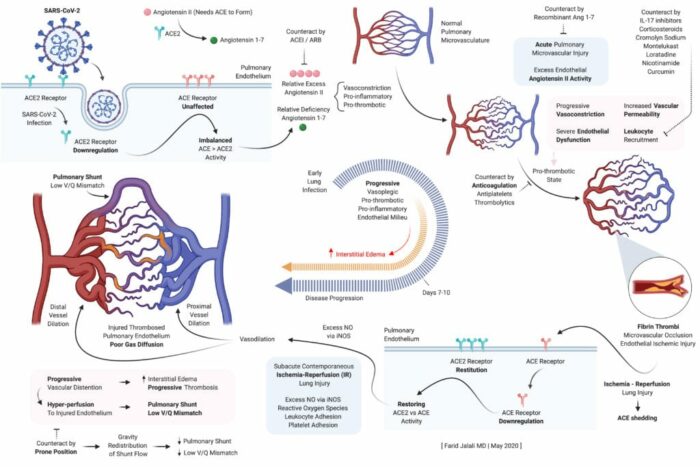
- Early endothelial stabilization, before hypoxia sets in, is key to prevent SARS-CoV-2 induced, excess Angiotensin II mediated, intense alveolar capillary vasoconstriction, as well as the concomitant pro-inflammatory, pro-thrombotic endothelial milieu, all of which form the basis of lung injury in COVID19.
- Alveolar capillary microvascular thrombi are not a pre-requisite for the severe lung injury in COVID19, but are a clear step in the wrong direction if allowed to be formed.
- Lung’s natural and physiologic protective response to SARS-CoV-2 induced alveolar capillary vasoconstriction and dead-space ventilation is characterized by alveolar hypocapnic bronchoconstriction at the level of the alveolar ducts to reduce a harmful alveolar expansion in these affected capillaries.
- Naturally, unaffected capillaries and corresponding alveoli will have a higher redistribution of ventilation, will exchange more CO2 into alveolar space, and will therefore have hypercapnic bronchodilation.
- This redistribution keeps lung compliance preserved in the initial lung injury characterized mainly by dead-space ventilation, forming intrapulmonary shunts, without significant interstitial or alveolar edema.
- Compensatory lower inspiratory volumes characterize the patient’s response, associated with higher respiratory rate, and “shallow rapid breaths” without distress. [this has not been my experience–EMCrit]
- This lower inspiratory volume is needed to prevent expansion of alveoli in the affected vasculopathic areas, as inappropriate expansion compounds the vasoconstriction in these affected alveolar capillaries.
- This will result in a compensatory tendency to develop hypocapnea on blood gas analysis, often concomitant with hypoxia as intrapulmonary shunts also begin to form as lung injury progress.
- Higher lung volumes, and positive pressure ventilation, disturb the fine balance maintained physiologically in the ventilatory redistribution pattern of the COVID1 9 lung, between high V/Q mismatch areas (poor perfusion, compensatory reduced ventilation to protect against the vasculopathy) and the compensating lower V /Q areas that safely receive higher ventilation in return.
- Therefore, mechanical ventilation may result in worsening of dead-space ventilation by constricting alveolar capillaries in the affected vasculopathic regions, and additionally result in worsening intrapulmonary shunting (next slide) due to reduced resistance in extra-alveolar vessels with higher lung volumes.
- In absence of endothelial stabilization, proper anticoagulation, and flow redistribution, lung Injury progresses to severe form by progressively worsening dead-space ventilation, resulting in intrapulmonary shunt development as described in the the diagrams.
- This advanced stage of lung injury is characterized by progressively diminished flow across the alveolar capillaries, resulting in higher flow across the formed intrapulmonary shunts, eventually culminating into progressive interstitial edema, progressive and diffuse alveolar damage, and alveolar fibrin thrombi deposition.
- Physiologically, this stage resembles “typical ARDS” where alveolar recruitment may be beneficial, but unlikely to reverse the vasculopathic disease process, inevitably resulting in high mortality. Pulmonary vasodilators and systemic vasoconstriction plausibly worsen hypoxia at this stage due to increasing flow across the intrapulmonary shunts.
- Through the action of body’s innate fibrinolytic system, lysis of microthrombi and reversal of flow to an area of injured endothelium may result in cycles of ischemia-reperfusion injury in the lung, mediated early on by monocytes and macrophages, and late by neutrophil activity.
- Reduction in leukocyte trafficking with corticosteroids and other therapeutics can be of value early on in the disease course to mitigate this ischemia-reperfusion injury.
- Late and sudden restoration of flow to a bed of alveolar capillaries that have had a prolonged and deep poor flow, usually in absence of proactive endothelial stabilization and proper anticoagulation, will inevitably result in a severe ischemia-reperfusion injury, significant interstitial and alveolar edema, and sudden demise.
- At this late of a stage in lung injury, ECMO may be the only solution available while pursuing lysis of microthrombi to restore alveolar capillary flow in a controlled fashion, while cardiopulmonary bypass is utilized to reduce risk of hemodynamic demise.
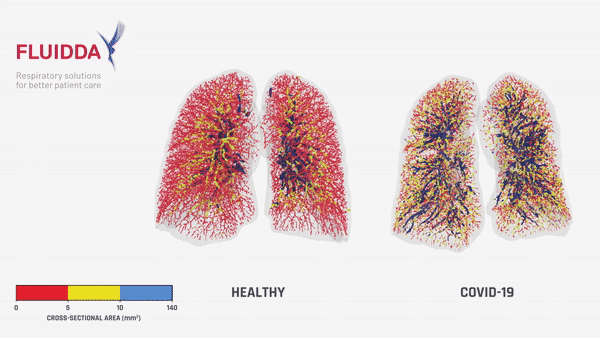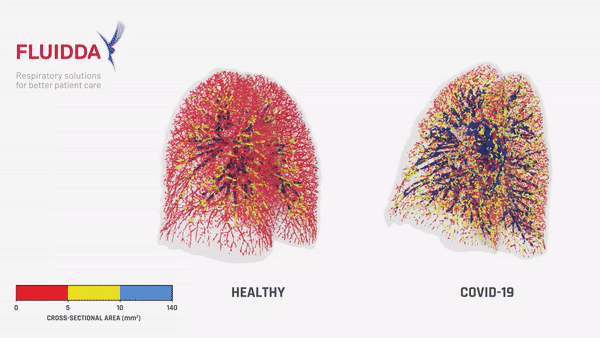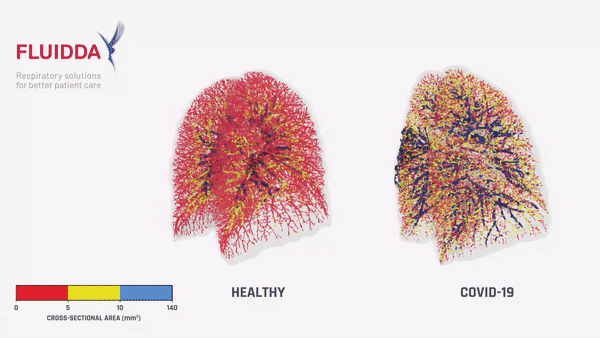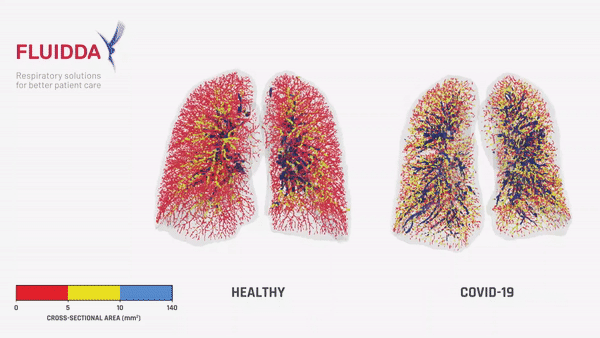
Fluidda data used in this theoretical model:
Below you can find data from FLUIDDA, collected with their FRI technology, used in the model. The graph illustrates that small vessels in COVID-19 (smaller than 5 mm2 cross sectional area) are significantly vasoconstrictor compared to normal, and even more so compared to “typical ARDS”. Moving to larger vessels, the COVID-19 begins to have a larger proportion of larger diameter vessels compared to healthy individuals (those sub-segmental arterioles seen on CT most likely).
Categorised in: Research / May 29, 2020 10:01 am /
Tags: COVID, COVID-19